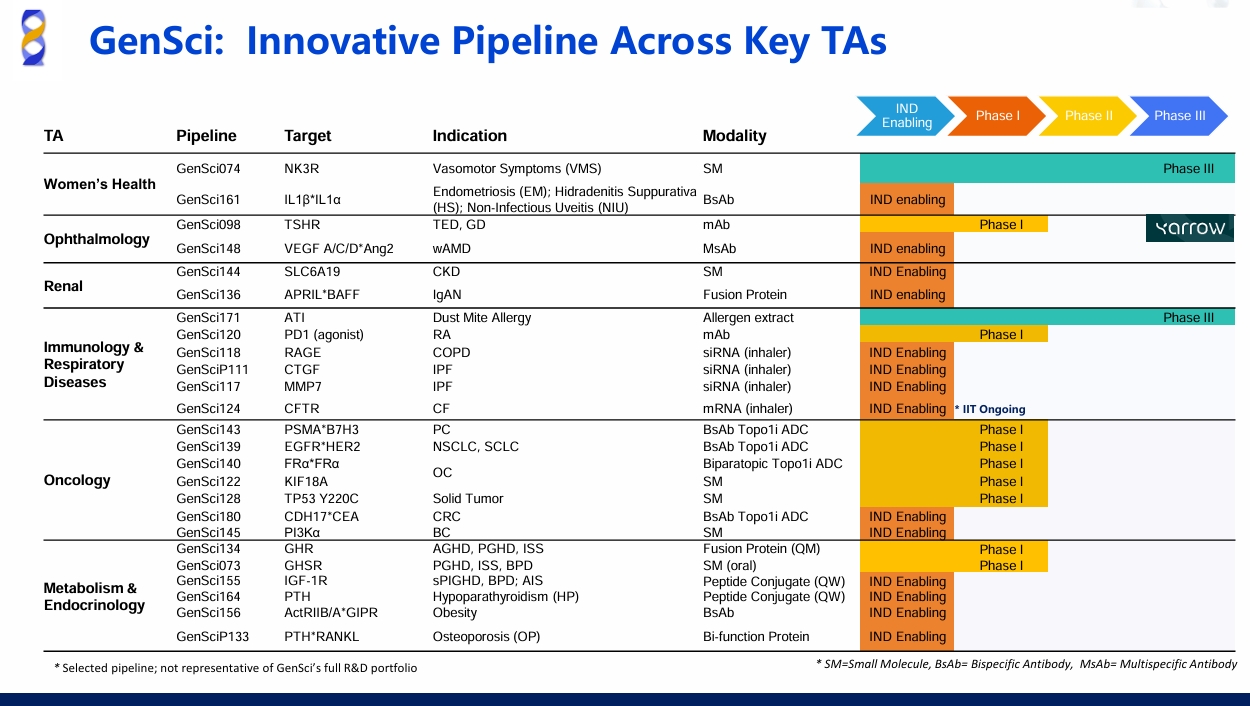
Firsekibart Approved with 87% Reduction in Gout Recurrence Over 6 Months
Time
2025-07-04
Readership
6942
Share



Recently, the National Medical Products Administration (NMPA) of China announced that Gensci's Class 1 innovative drug Jin Beixin Firsekibart has received approval. This drug is indicated for gouty arthritis (GA) treatment in adult patients for whom NSAIDs and/or colchicine are contraindicated, not tolerated, or ineffective and for whom repeated courses of corticosteroids are not suitable. Leveraging its clinical advantages of sustained symptom control, rapid onset of action and a favorable safety profile, Jin Beixin Firsekibart is expected to be China’s best-selling drug to address the unmet need in the treatment of autoimmune diseases.
Gout remains a prevalent disease affecting a growing number of patients. In China, patients with hyperuricemia (HUA) and with gouty arthritis (GA) are estimated at 177 million and 14.66 million respectively, making GA the second most common metabolic disorder after diabetes[1]. Despite the widespread use of conventional therapies in clinical practice, nearly 50% of gout patients still suffer from pain and other symptoms[2], and close to 60% experience recurrent gouty flares[3]. In particular, during the initial 3 to 6 months of urate-lowering therapy (ULT), nearly 12% to 61% experience recurrent gout flares, which significantly affects adherence and ultimately leads to ineffective treatment. Recurrent episodes of gout are associated with damage to joints and even pose additional health risks brought about by chronic inflammation involving the heart, the kidney and other critical organs[4,5].
The underlying cause of rucurrent gout flares is sustained inflammation, which significantly increases health risks[6-8]. Long-term anti-inflammatory therapy is essential to the treatment of GA and UTC as it helps reduce gout recurrence risks and protect target organs[8,9]. Therefore, there is an urgent need for a safer, more targeted and more sustained therapy that provides effective control of both inflammation and recurrence rate.
Firsekibart works through targeted inhibition of interleukin-1β (IL-1β), which is responsible for gouty inflammation. According to clinical trials, Firsekibart demonstrated rapid onset of action, providing pain relief equivalent to that of corticosteroids within 6 to 72 hours and reducing recurrence rate by 87%, with no serious adverse drug events[10].
Results from clinical trials also reveal that Firsekibart demonstrated non-inferiority to compound betamethasone in improving pain VAS scores within 72 hours. After a single dose, Firsekibart reduced the risk of first flare by 90% within 12 weeks, and by 87% within 24 weeks, compared with compound betamethasone. Regarding medication safety, no serious adverse drug events were observed with Firsekibart. These findings suggest that Firsekibart may be “best-in-class” among anti-inflammatory drugs for gout.
The approval of Jin Beixin Firsekibart as China’s first IL-1β monoclonal antibody (mAb) is expected to fill the gap in sustained and targeted anti-inflammatory therapy in the treatment of GA. In addition to its effect on relieving pain inflicted by gouty flares, chronic inflammation treatment with the IL-1β mAb, according to research, is also beneficial for the cardiovascular system, joints and respiratory system. In addition, IL-1β holds therapeutic potential in the treatment of metabolic disorders and oncologic diseases[11-14].
References:
[1] https://www.szyy.com.cn/sys-nd/1340.html
[2] 高维琴, 等. 痛风达标治疗人群用药真实情况分析:多中心真实世界研究结果[J]. 中华风湿病学杂志,2023,27(06):361-367.
[3] Fenando A, et al. Gout[M]. Updated 2024 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. https://www.ncbi.nlm.nih.gov/sites/books/NBK546606/
[4] 中华医学会内分泌学分会. 中国高尿酸血症与痛风诊疗指南(2019)[J]. 中华内分泌代谢杂志, 2020, 36(01):1-13.
[5] 徐东, 等. 痛风诊疗规范[J]. 中华内科杂志,2023,62(9):1068-1076.
[6] Jiang N, et al. NLRP3 Inflammasome: A New Target for Prevention and Control of Osteoporosis?[J]. Front Endocrinol (Lausanne). 2021 Sep 27;12:752546.
[7] Luo Z, et al. Role of microRNA alternation in the pathogenesis of gouty arthritis[J]. Front Endocrinol (Lausanne). 2022;13:967769.
[8] Ourada T, et al. Intercritical Gout Represents a Systemic Inflammatory State [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). Accessed November 6, 2024.
[9] Becker MA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout[J]. N Engl J Med. 2005 Dec 8;353(23):2450-61.
[10] Xue Y, et al. Efficacy and Safety of Genakumab versus Compound Betamethasone in Gout: The GUARD-1 Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9).
[11] Ridker PM, et al. CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease[J]. N Engl J Med. 2017;377(12):1119-1131.
[12] Everett BM, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure[J]. Circulation. 2019;139(10):1289-1299.
[13] Zhao R, et al. A critical role for interleukin-1β in the progression of autoimmune diseases[J]. Int Immunopharmacol. 2013 Nov;17(3):658-69.
[14] Syed Ali Wijdan, et al. The role of interleukin-1 beta in inflammation and the potential of immune-targeted
therapies[J].doi.org/10.1016/j.prerep.2025.100027.https://www.sciencedirect.com/science/article/pii/S2950200425000011