GenSci 2025 R&D Day: The Next Growth Frontier for a Billion-Dollar Growth Hormone Leader
Time
2025-05-20
Readership
6793
Share



On May 17,2025, GenSci successfully hosted its second R&D Day in Shanghai. The event brought together over 200 distinguished experts from academia and industry to explore GenSci's R&D strategy, technological innovation, clinical value translation, and global expansion. Combining on-site and virtual participation, the event attracted more than 22,000 global viewers via livestream, marking a milestone in fostering thought leadership across the biopharmaceutical ecosystem.

Scene of GenSci's 2025 R&D Day
01 From Single Focus to Multidimensional Innovation: Building GenSci's Next-Generation Product Portfolio

Dr. Lei Jin, General Manager of Changchun High-Tech Industry (Group), Founder, General Manager, and Chief Scientist of GenSci
Dr. Lei Jin outlined GenSci's global R&D strategy, emphasizing the company's evolution from a growth hormone pioneer to a diversified innovation powerhouse. "Technological innovation remains the cornerstone of transformative change, and I firmly believe in the power of innovation."He emphasized, "Through relentless R&D investment and cutting-edge platforms, we aim to establish new breakthroughs, fueling the company's sustainable and high-quality growth."
Women's and Children's Health: A Holistic Approach
GenSci has established a full lifecycle product matrix in pediatric and maternal health. Building on its leadership in recombinant human growth hormone (rhGH), the company now spans pediatric neurology, immunology, and respiratory care, while advancing pipelines in metabolic disorders and rare diseases. In women's health, GenSci's portfolio includes Jin Sai Heng® (the world's most comprehensive recombinant human follicle-stimulating hormone series) and Jin Sai Xin® (China's first water-soluble progesterone formulation). Future initiatives target infertility and gynecological infections, reinforcing GenSci's first-mover advantage.
Dr. Jin highlighted groundbreaking advancements across oncology, respiratory, and immunology. Megaxia® (nanocrystal megestrol acetate), China's sole therapy for cancer-related anorexia-cachexia syndrome, has been endorsed as a Category I recommendation in the CSCO guidelines, addressing a critical unmet need in supportive cancer care. In respiratory medicine, Jin Sai Ke®, the first pediatric Traditional Chinese Medicine (TCM) for acute cough caused by phlegm-heat syndrome , demonstrated a 74% cough resolution rate within 7 days in clinical trials, offering a novel therapeutic option for pediatric respiratory disorders. Meanwhile, Genakumab (anti-IL-1β monoclonal antibody), a long-acting biologic for acute gouty arthritis, is nearing commercialization with dual benefits of rapid pain relief within 6 hours and 87% reduction in recurrence risk within 6 months (HR=0.10, *p*<0.001), positioning it as a potential best-in-class therapy. Looking ahead, GenSci plans to expand its footprint in oncology, immunology, neuroscience, and rare diseases over the next five years, supported by strategic collaborations with global pharmaceutical leaders and academic institutions to accelerate innovation and clinical translation.
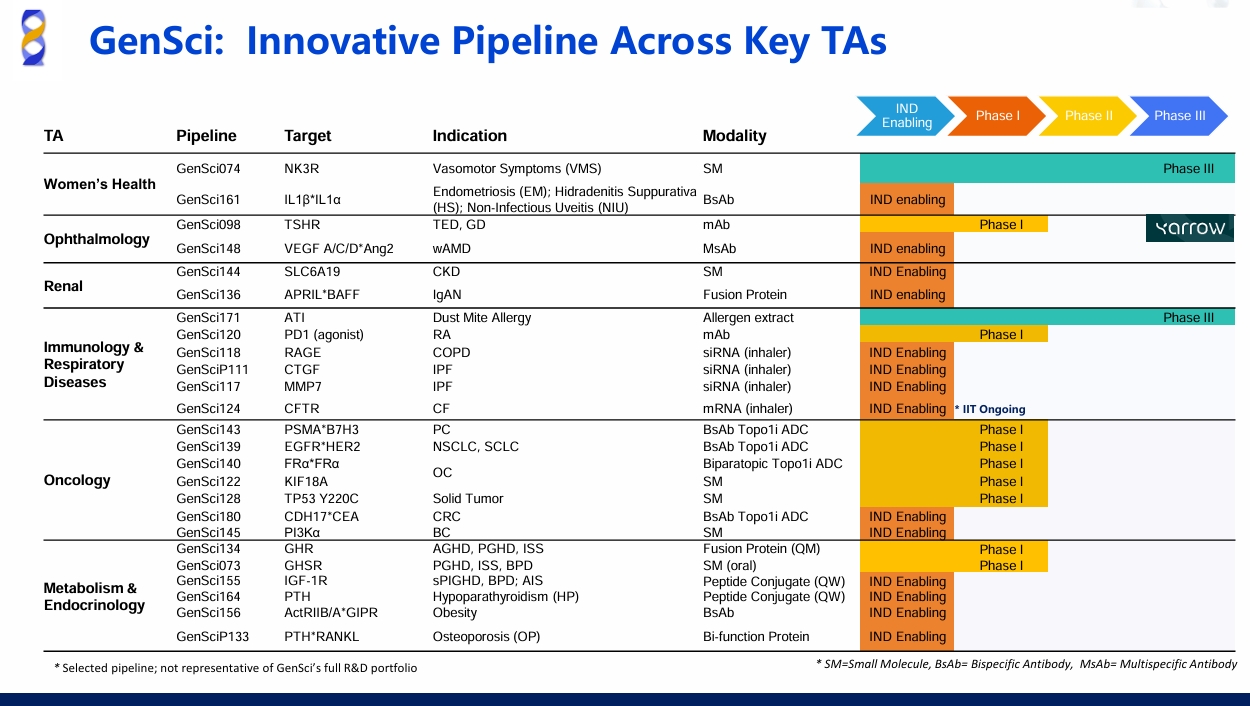
02 Endocrinology, Immunology and Oncology: FIC/BIC Pipelines in Focus
GenSci's 2024 R&D expenditure reached a new record high, marking 12 consecutive years of double-digit growth. Leveraging cutting-edge platforms such as protein sustained-release, antibody-drug conjugates (ADCs), siRNA, and live biotherapeutics, the company is accelerating the development of diversified pipelines spanning biologics, small molecules, and medical nutrition. Key breakthroughs include: the bispecific antibody-drug conjugate candidates GenSci143/GenSci139 (planned IND submission in oncology), the PD-1 agonist GenSci120 and APRIL/BAFF dual-target antagonist GenSci136 (showing Best-in-Class potential in autoimmune diseases), the NK3R antagonist GenSci074, a novel therapy demonstrating First-in-Class potential by targeting unmet needs in women's health, and the clinical-stage TSHR monoclonal antibody GenSci098 (ophthalmology). These milestones underscore GenSci's leading advantages in target innovation, clinical translation, and global competitiveness.
03 Genakumab: Phase III Data Reinforce Best-in-Class Potential
With China's gout prevalence exceeding 14 million patients, Genakumab (anti-IL-1β mAb) addresses critical unmet clinical needs. Professor Yu Xue from Huashan Hospital presented Phase III results demonstrating its best-in-class potential: compared to dexamethasone, Genakumab achieved a 90% reduction in recurrence risk at 12 weeks (*HR=0.10, p<0.0001*) and sustained an 87% risk reduction at 24 weeks (*HR=0.13, p<0.0001*), setting a new efficacy benchmark for IL-1β inhibitors. The drug has submitted a New Drug Application (NDA) to China's Center for Drug Evaluation (CDE), with expanded indications under active exploration, including systemic juvenile idiopathic arthritis (sJIA) and endometriosis. “Genakumab's dual benefits in rapid symptom control and long-term recurrence prevention position it as a transformative therapy for inflammatory diseases,” emphasized Prof. Xue.

Prof. Yu Xue, Deputy Director of Rheumatology, Huashan Hospital
The event included roundtables on globalization strategies, where experts from multinational corporations, clinicians, and investors emphasized the importance of strengthening registrational and investigator-initiated trials (IITs) to generate robust evidence, pioneering high molecular-weight drugs and protein degraders, and building multidisciplinary care networks to enhance disease management.
04 Innovation as the Core, Excellence as the Goal
GenSci is accelerating its transformation from a single-therapy leader to a multidimensional innovator. With a patient-centric approach and global partnerships, the company aims to deliver high-impact therapies worldwide.
Looking Ahead: GenSci will continue scaling R&D investments, advancing strategic pipelines, and expanding its international footprint to benefit patients across borders.