GenSci Partners with Allergy Immunotherapy Leader ALK to Bring Innovative AIT Solutions for Chinese Patients
Time
2025-09-18
Readership
2570
Share



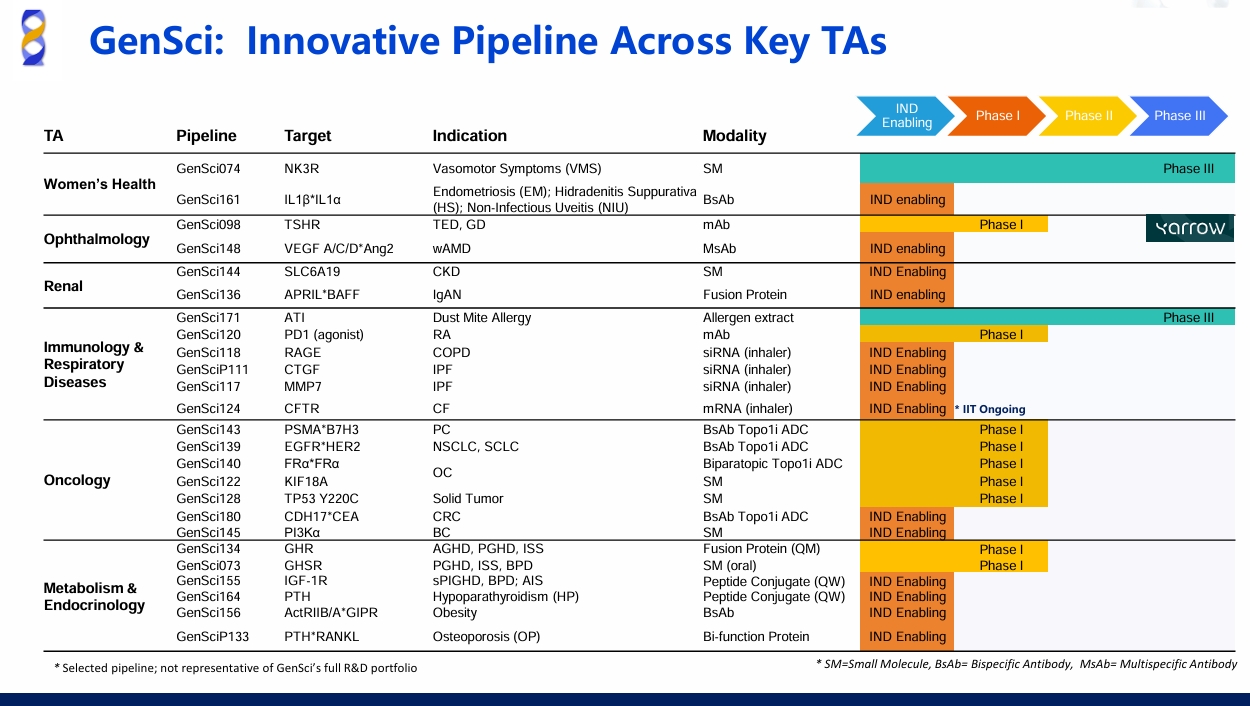
On September 17th, 2025, Shanghai, China -- Changchun GeneScience Pharmaceutical Co., Ltd. ("GenSci"), announced a partnership agreement with Abelló A/S ("ALK"), the Denmark-based global leader in allergy immunotherapy (AIT). Under the agreement, GenSci and ALK will work closely together to develop and commercialise ALK's AIT products in China; meanwhile, GenSci will obtain exclusive rights to distribute 3 AIT products developed by ALK in mainland China.

CEOs of GenSci and ALK entered into a strategic cooperation agreement
The products involved in this cooperation include 2 AIT products and 1 skin prick test kit for allergen diagnosis developed by ALK. Under the terms of the agreement:
GenSci is granted exclusive rights to ALK's portfolio of AIT products, including the injectable Alutard®house dust mite ("HDM") product, skin prick tests, and the ACARIZAX(®) SLIT-tablet in mainland China until December 31st, 2039.
From October 1st, 2025, GenSci will take over the sales and marketing of ALK's approved collaborative products in mainland China. The two parties will jointly continue ongoing clinical development of ACARIZAX® SLIT-tablet to facilitate its approval for adult and adolescent indications. Following the approval by the NMPA, GenSci will manage the sales and marketing of ACARIZAX® in mainland China.
According to the report by Fortune Business Insights, the global AIT market is projected to reach USD 3.2 billion by 2030, with a compound annual growth rate (CAGR) of 9.5%. China has the largest population suffering from HDM allergy, yet the market remains significantly underpenetrated with fewer than 1 million patients currently receive allergen immunotherapy (AIT), predominantly in the form of sublingual drops--especially among children, who bear a particularly high disease burden. With limited approved immunotherapy options available, substantial unmet clinical needs persist.
AIT is the only treatment capable of modifying the course of the disease. It stimulates an immune response in the body, which causes the patient to build up immunological tolerance against the given allergen. Alutard®, a long-established, globally marketed AIT therapy for HDM allergy, has demonstrated outstanding efficacy and gained recognition from both patients and experts. Another product — ACARIZAX® delivers AIT via sublingual immunotherapy (SLIT) and has been approved in multiple European and American markets. The approvals were based on the largest ever clinical development programme with house dust mite allergy immunotherapy involving more than 9,000 subjects. It is supported by evidence-based data and offers a convenient, effective, and safe therapeutic option.
With a century of leadership in allergy immunotherapy and a market share of over 45%, ALK, headquartered in Denmark and listed on NASDAQ Copenhagen, has pioneered in the field of respiratory allergies. GenSci is a leading biopharmaceutical company in China with extensive experience in the pediatric field, strong presence in key therapeutic areas such as respiratory and immunology, with substantial expertise in clinical development and commercial operations. Moving forward, GenSci and ALK will collaborate closely to accelerate the commercialization, ensuring these products reach and benefit a broader patient population.

Peter Halling, CEO, Abelló A/S
Commenting on the partnership, ALK's CEO Peter Halling says: "We're very excited about the prospects of speeding up market uptake of ALK's evidence-based AIT products in China. We partner for strength, and GenSci has a well-proven track record of navigating this important market and intends to deploy significant resources, including a sizeable sales force, to succeed with our AIT products. We see a strong strategic fit and look forward to co-operating with GenSci to benefit even more patients and healthcare professionals. "

Dr. Lei Jin, General Manager of Changchun High-Tech Industry (Group),
Founder, General Manager, and Chief Scientist of GenSci
Dr. Jin Lei, Founder, General Manager & Chief Scientist of GenSci, General Manager of Changchun High-Tech, stated, "We're thrilled to partner with ALK. Through this cooperation, ALK will deepen its presence in China's AIT market, and we will enrich our diversified product portfolio. The ALK products will trengthen our existing product portfolio and R&D pipeline and enhance our overall competitiveness in this field. We see vast unmet clinical needs as China has the world's largest allergic patient population. We will work closely with ALK to improve access to products like Alutard®, and deliver safer, more convenient, and efficient treatment solutions to patients."
About ALK
ALK is a global specialty pharmaceutical company focused on allergy and allergic asthma. ALK manufactures and markets allergy immunotherapy (AIT) treatments and other products and services for people with allergy and allergy doctors. Headquartered in Hørsholm, Denmark, ALK employs around 2,800 people worldwide and is listed on Nasdaq Copenhagen. Find more information at www.alk.net.
About Changchun GeneScience Pharmaceutical Co., Ltd. (“GenSci”)
GenSci is a leading biopharmaceutical company in China, specialising in pediatric and women's health. Additionally, GenSci is active in four other therapeutic areas: Endocrinology, Metabolic, Immunology/Respiratory, and Oncology. The company has over 9,000 employees, and integrates research, development, production, and marketing of innovative therapies in its value chain. Established in 1997, the company is a subsidiary of Changchun High-Tech Industries (Group) and is headquartered in Changchun, China.