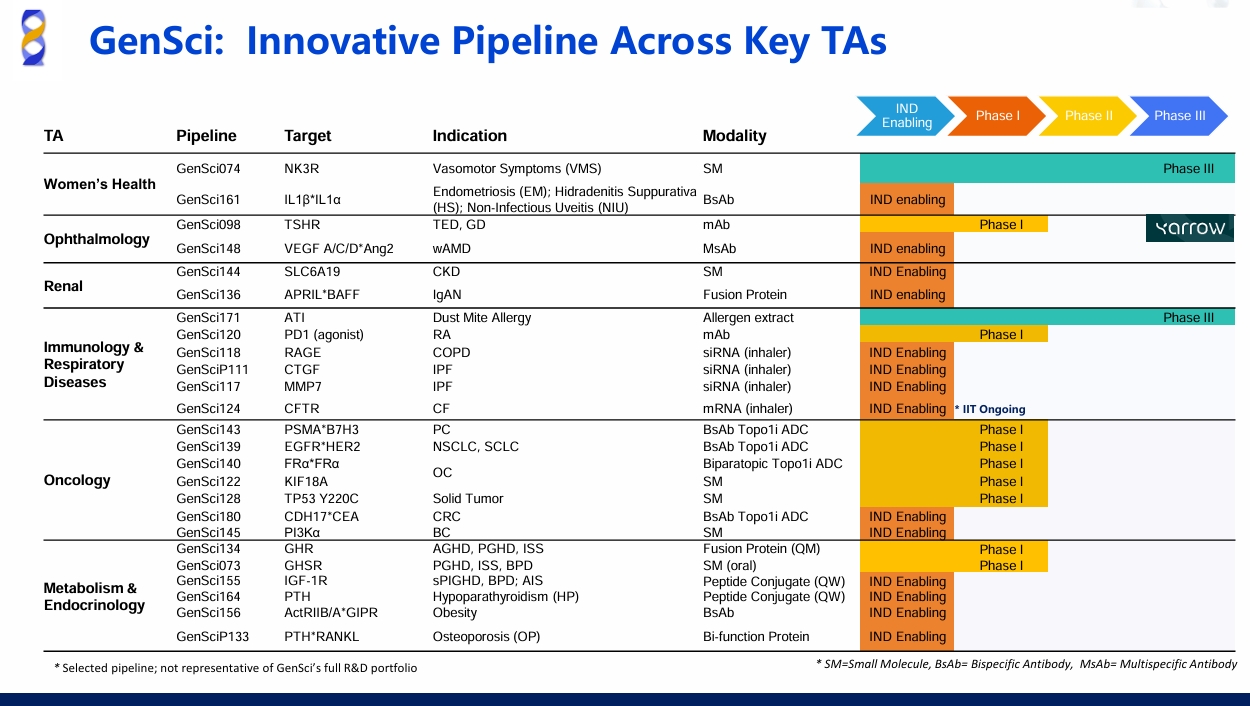
The FDA has Cleared the IND Application for GenSci128 for TP53 Y220C-Mutated Solid Tumors
Time
2025-05-30
Readership
12247
Share



On May 28, 2025 , Changchun GeneScience Pharmaceutical Co., Ltd. (GenSci) announced that GenSci128 has officially received clearance from the U.S. FDA, which is indicated for locally advanced or metastatic solid tumors with TP53 Y220C mutation.
The TP53 gene is the most frequently mutated tumor suppressor gene in cancer, and its encoded p53 protein acts as a transcription factor with tumor-suppressive functions ,inactivates p53 and drives tumor development.
As a reactivator of p53 Y220C, Gensci128 binds the mutant form and restores WT p53 tumor-suppressive activity by normalizing the conformation of the TP53 Y220C mutant protein, enhancing its stability, and reactivating its transcriptional function.Preclinical data demonstrate that GenSci128 exhibits promising efficacy and safety profiles.
GenSci128 has also obtained IND clearance from China's NMPA. GenSci will conduct parallel clinical studies in China and the U.S., dedicated to delivering innovative treatment options to patients globally.