A Leading Force in Innovation:
GenSci Earns Dual Recognition as Global Innovator & 2025 China Pharma Top 100
Time
2025-12-02
Readership
3144
Share



GenSci, a subsidiary of Changchun High-tech, has recently been honored on two authoritative pharmaceutical innovation rankings, underscoring its leadership in China's innovative drug development and global expansion.
At the Business & Philanthropy Forum 2025 in Singapore, iiMedia Research released the 2025 China Leading Enterprises Ranking for Innovative Drug Overseas Expansion list. Driven by its strong R&D capabilities and global expansion strategy, GenSci ranked 25th, reflecting its growing international presence and robust R&D capabilities. Meanwhile, ranked in the top tier of China's 2025 Top 100 Innovative Pharmaceutical Companies lately, Changchun High-tech reinforces its status as an innovation leader with distinct competitive edges.

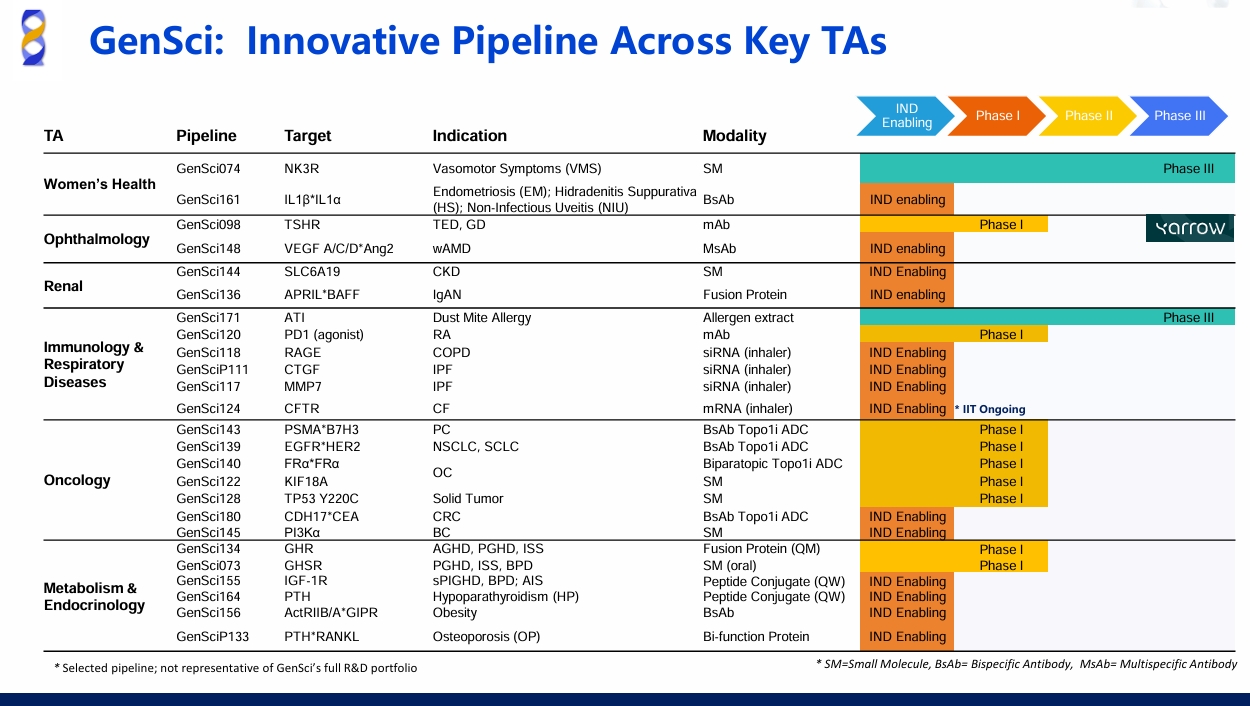
As a pioneer in China's innovative drug sector, GenSci continues to ramp up R&D investment. The company has established cutting-edge technology platforms, including small molecule, tissue-targeted siRNA, antibody drug conjugates (ADCs), molecular glue, long-acting and slow effective release, and radioligand therapy (RLT). Currently, GenSci has over 40 clinical candidates, including more than 10 in Phase III or NDA stage, 5 receiving IND approvals from the FDA, and more than 10 innovative drugs. Many candidates have first-in-class (FIC) or best-in-class (BIC) potential across various therapeutic areas. Highlights include:
· Women's Health : GenSci074 NK3R antagonist for vasomotor symptoms (VMS) in menopause (hot flashes) has advanced into Phase III clinical trials.
· Oncology : Multiple ADCs, including GenSci143 (a BsADC targeting B7-H3/PSMA), GenSci139 (a BsADC targeting EGFR/HER2), and GenSci140 (a biparatopic ADC targeting FRα), are in clinical development.
· Endocrinology : Advancement has been made in GenSci134, a once-monthly long-acting growth hormone (GH), and GS3-007a, China’s first oral small-molecule GHSR-1a agonist, after the launch of its once-weekly long-acting rhGH (recombinant human growth hormone) in 2014.
As GenSci's ongoing efforts in translating drug discovery into accessible solutions, two innovative drugs were launched in China in 2025. Firsekibart, approved in June for gouty arthritis (GA), addresses significant unmet needs by delivering a long-acting, targeted therapy. Another product, Corifollitropin alfa N02 Injection, a long-acting FSH injection approved in September, offers a once-weekly regimen that significantly improves patient experience in assisted reproduction.
To accelerate its global pace, GenSci partners with multinational pharmaceutical companies, biotech firms, and global investment organizations. The company is also extending the global reach of its established portfolio and increasing its presence in the global market. From pioneering long-acting GH therapy to building a diversified, differentiated pipeline across multiple therapeutic areas, GenSci remains committed to innovation. Moving forward, it will continue to bring more innovative therapies to patients worldwide.