GenSci Unveils 7 Innovative Pipeline at JPM 2026
Time
2026-01-16
Readership
5565
Share



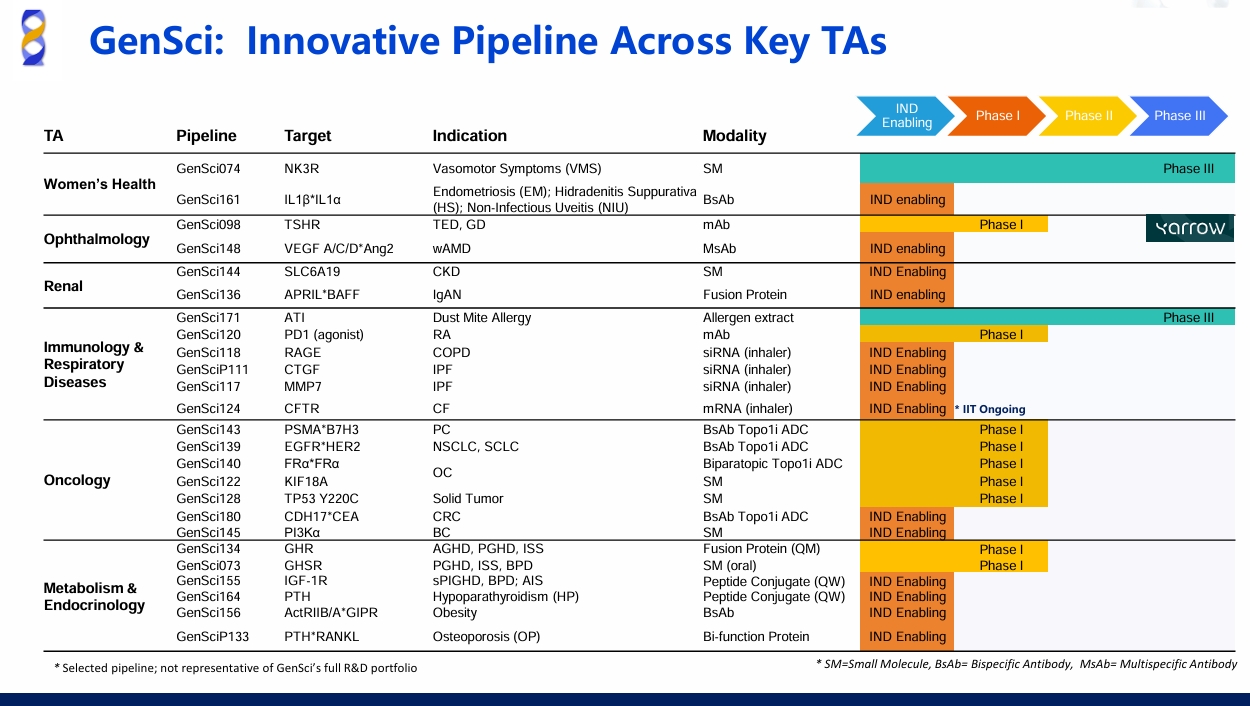
SAN FRANCISCO—J.P. Morgan Healthcare Conference—Jan 13, 2026—Changchun GeneScience Pharmaceutical Co., Ltd. ("GenSci") announced that GenSci was invited to the 44th Annual J.P. Morgan Healthcare Conference (JPM). At the conference, Dr. Yuanfeng Xia, Vice President of GenSci and Head of R&D Business Development and License-Out, delivered a presentation themed Innovative Drug R&D at GenSci, in which GenSci’s innovation platforms and 7 priority pipeline assets were overviewed.
GenSci also highlighted its core technology platforms. GenSci155 (IGF-1) and GenSci164 (PTH), developed on the long-acting & sustained release platform, demonstrate the potential to achieve the longest dosing intervals worldwide. GenSci111 and GenSci117, enabled by the siRNA with targeted delivery platform, achieved highly specific target knockdown in lung tissue while significantly reducing off-target exposure in extra-pulmonary organs such as the kidney, thereby improving safety profiles. Dual-target ADC candidates GenSci139 and GenSci143, which exhibit excellent plasma stability, potent anti-tumor activity, and wide therapeutic windows, following optimization of GenSci’s antibody-drug conjugates (ADC) platform.
As a pioneer in China’s growth hormone (GH) therapeutics, GenSci continues to strengthen its leadership in endocrinology. Its monthly dosing therapy, GenSci134, has entered Phase I clinical trial for GH deficiency and related growth disorders. It's expected to significantly improve patient convenience and adherence.
In women’s health, GenSci074, an NK3R antagonist for the treatment of menopausal vasomotor symptoms (VMS), successfully met all primary endpoints in its China Phase II study. Clinical results demonstrated statistically significant improvements in both frequency and severity of hot flash at Weeks 4 and 12 compared with placebo. GenSci074 showed a favorable safety profile, with adverse event rates similar to placebo and no observed liver toxicity signal or NK1R-related neurological side effects. GenSci074 is planned to initiate the Phase III MRCT in Q2 2026. For 800 million postmenopausal women worldwide affected by VMS, GenSci074 offers potential for Best-in-Class therapy.
In respiratory and immunology, GenSci120, a PD-1 agonist with Best-in-Class potential, has received IND approval in both China and the United States. Clinical data demonstrated full receptor occupancy, with PD-1high T cell depletion. PK/PD data further support the potential for monthly dosing. As a novel mechanism, GenSci120 is expected to offer a new solution for immune-inflammatory diseases. A Phase IIb study in rheumatoid arthritis(RA) is currently in preparation, with additional approved indications including systemic lupus erythematosus (SLE) and primary Sjögren’s syndrome (pSS).
In oncology, GenSci128, a corrector targeting TP53 Y220C mutation, has entered clinical development. TP53 mutation exists in multiple cancers without approved therapies globally. GenSci128 demonstrated proof of mechanism in patients with advanced solid tumors harboring TP53 Y220C mutation and has demonstrated favorable efficacy and safety in preclinical studies. GenSci145, a selective inhibitor of PI3Kα mutant, received IND approval from the NMPA in December 2025. Designed for patients with locally advanced or metastatic solid tumors harboring PI3Kα mutations, GenSci145 exhibits selective inhibition across multiple PIK3CA mutations, improved brain penetration, and no hyperglycemia effect, positioning it as a potential treatment option for subcutaneous and brain orthotopic tumor.

Innovative assets presented at JPM
In addition, GenSci also showcased other pipeline assets with Best-in-Class or First-in-Class potential, including GenSci133, a PTH–RANKL antibody fusion protein for osteoporosis and other bone-related diseases, and GenSci144, a SLC6A19 small molecule inhibitor for phenylketonuria (PKU). Both candidates are expected to enter clinical development in 2026.
JPM connects global industry leaders, emerging fast-growth companies, innovative technology creators and members of the investment community. At this premier conference, more than 20 leading Chinese biopharma companies drew the world's attention with their innovative assets. At JPM, GenSci shows its differentiated innovation capabilities to global partners and advances collaboration to accelerate global development and commercialization.