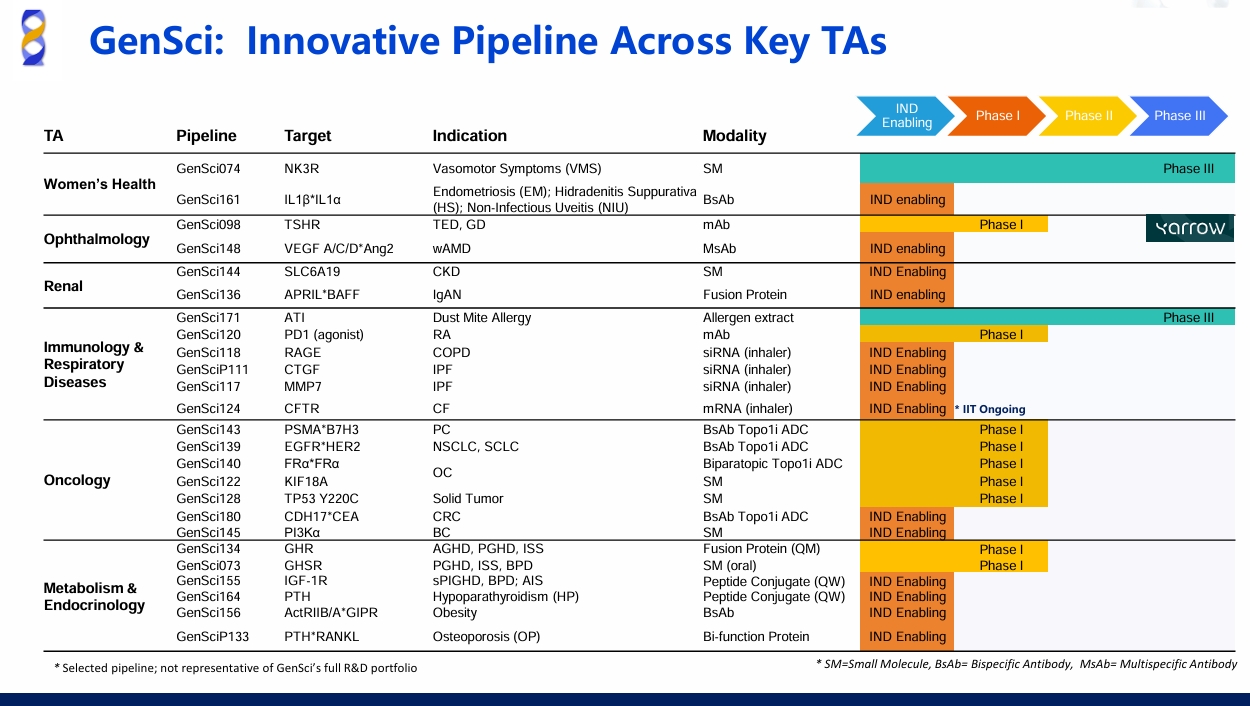
NMPA Approves the PD-1 agonist GenSci120 for Clinical Trials Commencement
Time
2025-01-17
Readership
5729
Share



On January 15, 2025, Changchun GeneScience Pharmaceutical Co., Ltd. (GenSci) received approval from the National Medical Products Administration (NMPA) for its independently developed humanized antibody, the PD-1 agonist GenSci120, to proceed with clinical trials including adult systemic lupus erythematosus and adult primary Sjögren’s syndrome.
Globally, over a dozen PD-1 agonists are in development, with most still in preclinical stages. The preclinical studies have demonstrated that GenSci120 injection offers a relatively specific targeted immunosuppressive effect, highlighting its potential as a therapeutic option for autoimmune diseases.
Dr. John Xu, GenSci's Executive Vice President of Immunology and Oncology Drug Development and a key inventor of GenSci120, stated: "Preclinical data shows GenSci120’s Best-in-Class potential, giving us confidence to explore its use across multiple indications."