Clinical Trial Commencement of GenSci120 Injection Approved
Time
2025-02-10
Readership
4530
Share



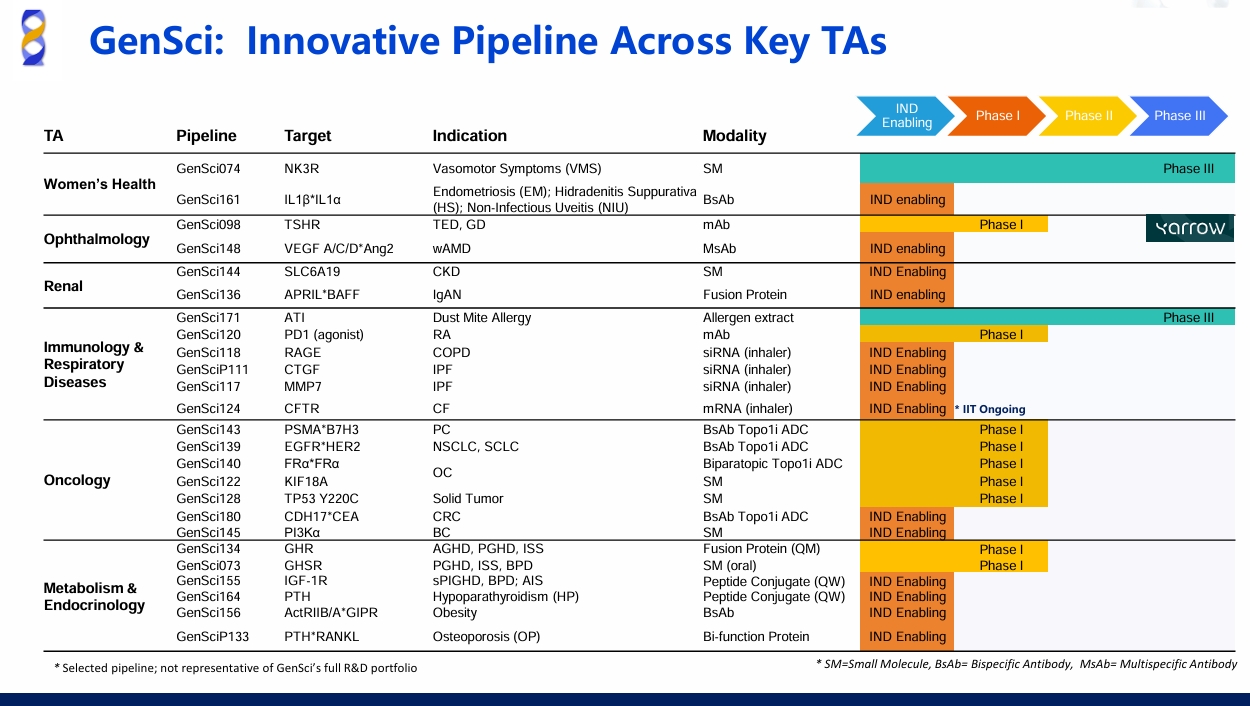
Recently, Changchun GeneScience Pharmaceutical Co., Ltd. (GenSci) announced that the National Medical Products Administration (NMPA) has approved an IND application for the PD-1 agonist GenSci120 for the treatment of Rheumatoid Arthritis.
GenSci120 is a Category 1 new biologic drug (equal to "BLA"). Preclinical studies have shown that GenSci120 injection has promising therapeutic potential for autoimmune diseases. This approval will facilitate further clinical development of the product and help address the unmet medical needs of patients.
Additionally, GenSci120 is currently undergoing clinical research for other autoimmune diseases including Adult Systemic Lupus Erythematosus, Adult Primary Sjögren's Syndrome, Inflammatory Bowel Disease, and Rheumatoid Arthritis.