GenSci Announces Positive Phase II Results for GS1-144 Tablets in Chinese Postmenopausal Women with Moderate-to-Severe VMS
Time
2025-10-24
Readership
65
Share



· GS1-144 reduced both the frequency and severity of moderate-to-severe VMS in Chinese postmenopausal women.
· The 60 mg QD and 30 mg BID regimens delivered the clearest and most consistent efficacy signals at Week 12.
· The safety profile observed over 12 weeks was acceptable and generally comparable to placebo on the key measures.
· The positive data will support further clinical development.
China — Oct. 24, 2025 — Changchun GeneScience Pharmaceutical Co., Ltd. (GenSci) announced topline and detailed results from GenSci074‑201, a Phase II clinical trial evaluating GS1-144 tablets for the treatment of moderate‑to‑severe vasomotor symptoms (VMS) — hot flashes and night sweats — in Chinese postmenopausal women. The study met its prespecified co-primary endpoints and demonstrated clinically meaningful reductions in both frequency and severity of moderate‑to‑severe VMS for two active regimens (GS1-144 60 mg once daily and GS1-144 30 mg twice daily) versus placebo, while maintaining a generally favorable safety and tolerability profile, without risk of liver safety.
The GenSci074-201 trial is a multicenter, randomized, double-blind, placebo-controlled Phase 2 study conducted at 46 sites in China, led by Peking Union Medical College Hospital. It enrolled 276 postmenopausal women experiencing ≥7 moderate-to-severe VMS daily. Participants were randomized to four groups: GS1-144 30 mg QD, 60 mg QD, 30 mg BID or placebo for 12 weeks.
GS1-144 demonstrated clear and clinically relevant effects on both frequency and severity of moderate-to-severe VMS.
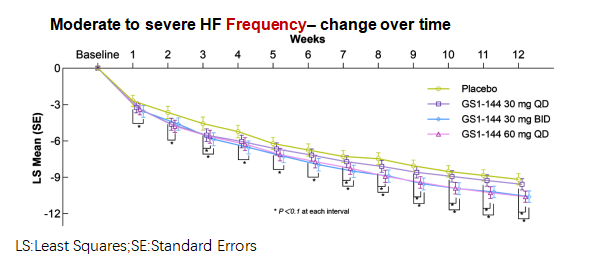
Week 4 frequency: The GS1-144 30 mg BID arm achieved a statistically significant greater reduction in mean daily frequency of moderate-to-severe VMS compared with placebo (least squares mean [LSM] difference −1.305 episodes/day; 90% CI −2.348, −0.263; p = 0.0398).
Week 12 frequency: Both GS1-144 60 mg QD and GS1-144 30 mg BID produced statistically significant reductions in mean daily frequency versus placebo at Week 12. The LSM difference was −1.418 episodes/day for 60 mg QD (90% CI −2.283, −0.553; p = 0.0072) and −1.407 episodes/day for 30 mg BID (90% CI −2.277, −0.536; p = 0.0081). These differences indicate a clinically meaningful reduction compared with placebo and suggest a dose-response relationship across the arms.
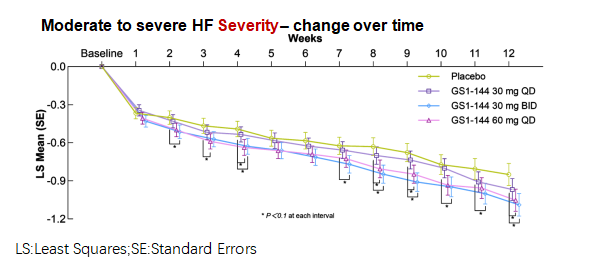
Severity outcomes: At Week4 and 12, both GS1-144 doses achieved a statistically significant reduction in mean severity of moderate to severe VMS versus placebo: the 30 mg BID arm (LSM difference −0.237; 90% CI −0.428, −0.046; p = 0.0418) and the 60 mg QD arm (LSM difference −0.192; 90% CI −0.382, −0.001; p = 0.0980).
GS1-144 was well tolerated in this study, with a safety profile that supports its further development. The incidence of treatment-emergent adverse events (TEAEs) was similar between pooled GS1-144 arms (67.6%) and placebo (62.3%). Most TEAEs were mild or moderate in severity. And no risks of liver enzyme elevation were observed.
Reference
1. TMS 2025. Efficacy and safety of GS1-144 in postmenopausal women with moderate-to-Severe vasomotor symptoms: A phase 2 clinical trial. P-192
About GS1-144
GS1-144 is a novel selective NK3R antagonist designed to modulate KNDy neuron activity in the hypothalamus and thereby normalize thermoregulatory signaling that contributes to hot flashes. GS1-144 is being developed as a nonhormonal therapeutic option for menopausal vasomotor symptoms.
Disclosure Notice
The information contained in this release is as of October 24, 2025. Gensci assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.








