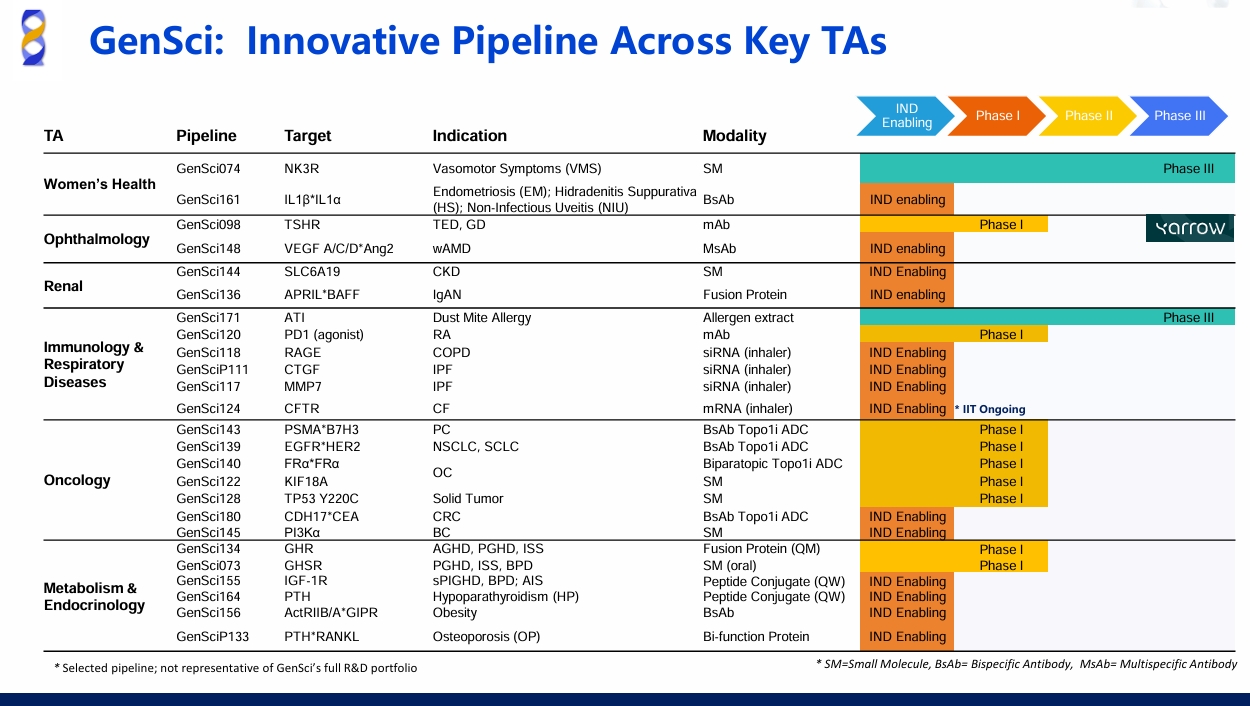
GenSci136, an APRIL/BAFF Dual Antagonist, Granted Clinical Trial Approval for IgAN
Time
2026-02-14
Readership
30
Share



SHANGHAI, China – Feb. 12, 2026 – Changchun GeneScience Pharmaceutical Co., Ltd. ("GenSci") recently announced that the National Medical Products Administration (NMPA) has approved an IND application of GenSci136 for Injection, a dual APRIL/BAFF antagonist in development for the treatment of Immunoglobulin A nephropathy (IgAN).
IgAN is the most common primary glomerulonephritis worldwide, including in China.Diagnosis typically occurs in patients aged 30 to 40, and while progression is often gradual, long-term outcomes remain poor. A 20-year follow-up study of a large cohort found that nearly 80% of IgAN patients progress to end-stage renal disease (ESRD) within two decades of diagnosis. Despite its slow clinical course, the disease is marked by complex pathophysiology and irreversible renal damage, positioning IgAN as a growing public health burden worldwide.
Current treatment remains largely supportive. Available pharmacological options aimed at reducing inflammation or suppressing the production of pathogenic IgA are limited by suboptimal efficacy and safety concerns, highlighting a significant unmet need for more targeted, disease-modifying therapies.
GenSci136 is a novel recombinant trimeric fusion protein engineered to mimic the native extracellular domain of B-cell maturation antigen (BCMA). By neutralizing both BAFF and APRIL, it potently blocks their interaction with cognate receptors on B cells and plasma cells, thereby modulating the survival, differentiation, and antibody production of pathogenic cell lineages. Through this targeted intervention in aberrant immune activation—central to the pathogenesis of IgAN—GenSci136 has the potential to reduce the generation of nephrotoxic antibodies at the source.
The molecule also incorporates an anti-HSA VHH domain designed to extend its half-life in vivo, a feature expected to support durable pharmacodynamic activity and less frequent dosing. With its dual mechanism of action and optimized pharmacokinetic profile, GenSci136 represents a potential first-line targeted therapy for the long-term management of IgAN.