-

2026-01-16
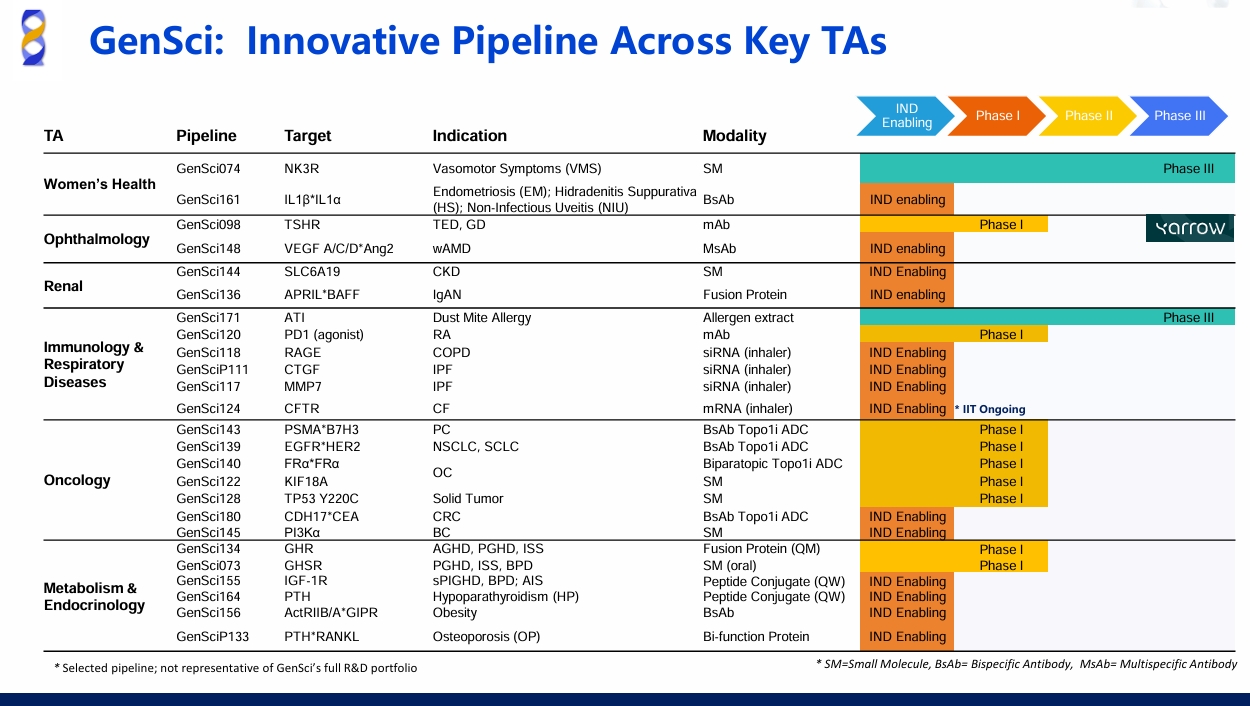
GenSci Unveils 7 Innovative Pipeline at JPM 2026Learn More
-


2025-12-23
GenSci Partners with Leading AI Company Partex AI to Accelerate Drug Development and Global CommercializationLearn More
-


2025-12-16
GenSci Secures Up to $1.365B in Global ex-China Licensing Deal for GS-098(YB-101) with RTW InvestmentsLearn More
-


2025-12-02
A Leading Force in Innovation:
GenSci Earns Dual Recognition as Global Innovator & 2025 China Pharma Top 100Learn More
-


2025-09-18
GenSci Partners with Allergy Immunotherapy Leader ALK to Bring Innovative AIT Solutions for Chinese PatientsLearn More
-

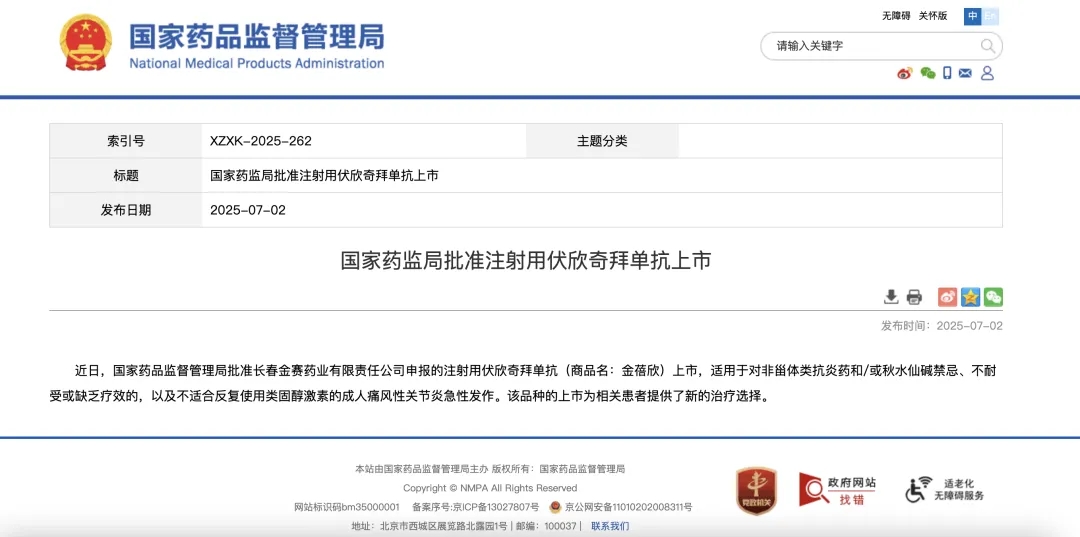
2025-07-04
Firsekibart Approved with 87% Reduction in Gout Recurrence Over 6 MonthsLearn More
-


2025-06-18
NMPA Approves New Indication Clinical Trial of Megestrol Acetate Oral SuspensionLearn More

-
2024-08-29
GenSci098 Injection Obtains Implied License For Clinical Trials of FDALearn More

-
2024-08-21
Jintrolong® New Dosage Form of Long-acting Somatropin Injection ApprovedLearn More

-
2024-08-06
Clinical Trial Commencement of GenSci098 Injection ApprovedPreclinical data indicate GenSci098 has the potential to become a new treatment for Thyroid Eye Disease
Learn More

-
2024-07-26
Zhejiang University – GenSci Joint Research Center of Children’s Health establishedLearn More

-
2024-07-23
NMPA: New Drug Application of Recombinant Human Follicle-Stimulating Hormone - CTP Fusion Protein Injection AcceptedThis product will potentially enhance convenience and patient compliance
Learn More

-
2024-07-09
R&D Innovation Accelerator: Shanghai Children's Hospital and GenSci Ink Strategic Cooperation AgreementTwo Sides will Collaboratively Set Up a R&D Center
Learn More











