-

2026-01-16
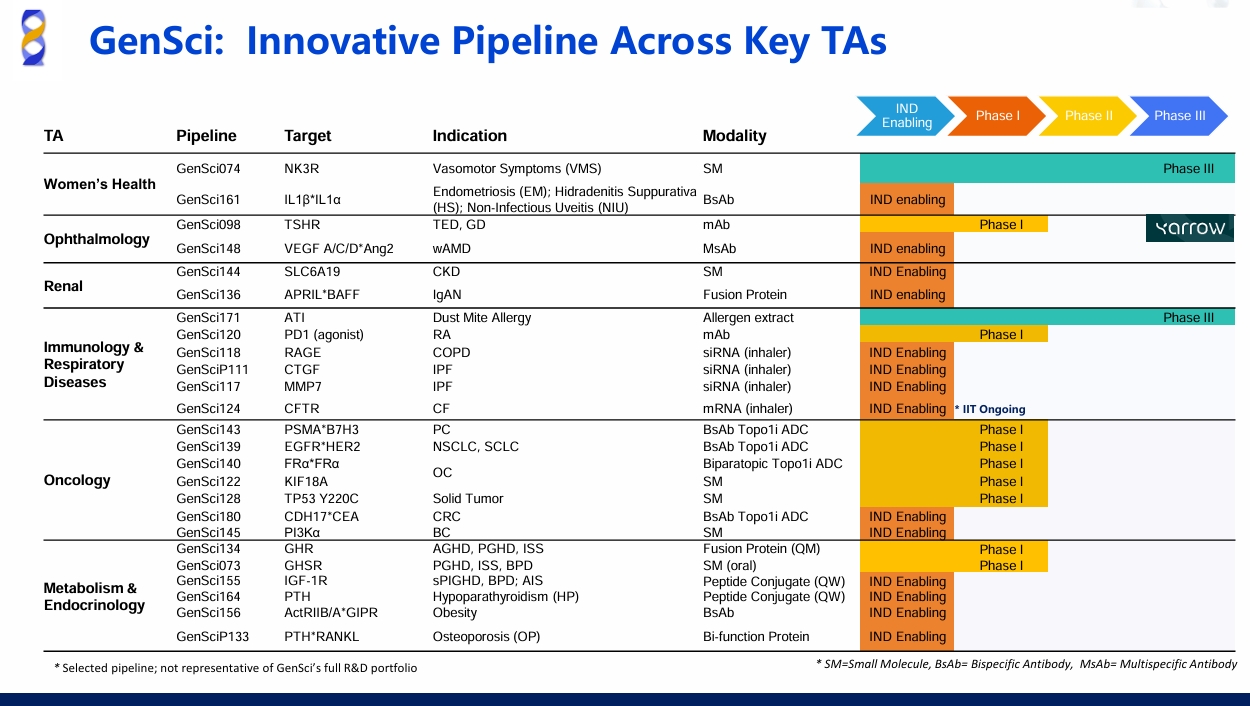
GenSci Unveils 7 Innovative Pipeline at JPM 2026Learn More
-


2025-12-23
GenSci Partners with Leading AI Company Partex AI to Accelerate Drug Development and Global CommercializationLearn More
-


2025-12-16
GenSci Secures Up to $1.365B in Global ex-China Licensing Deal for GS-098(YB-101) with RTW InvestmentsLearn More
-


2025-12-02
A Leading Force in Innovation:
GenSci Earns Dual Recognition as Global Innovator & 2025 China Pharma Top 100Learn More
-


2025-09-18
GenSci Partners with Allergy Immunotherapy Leader ALK to Bring Innovative AIT Solutions for Chinese PatientsLearn More
-

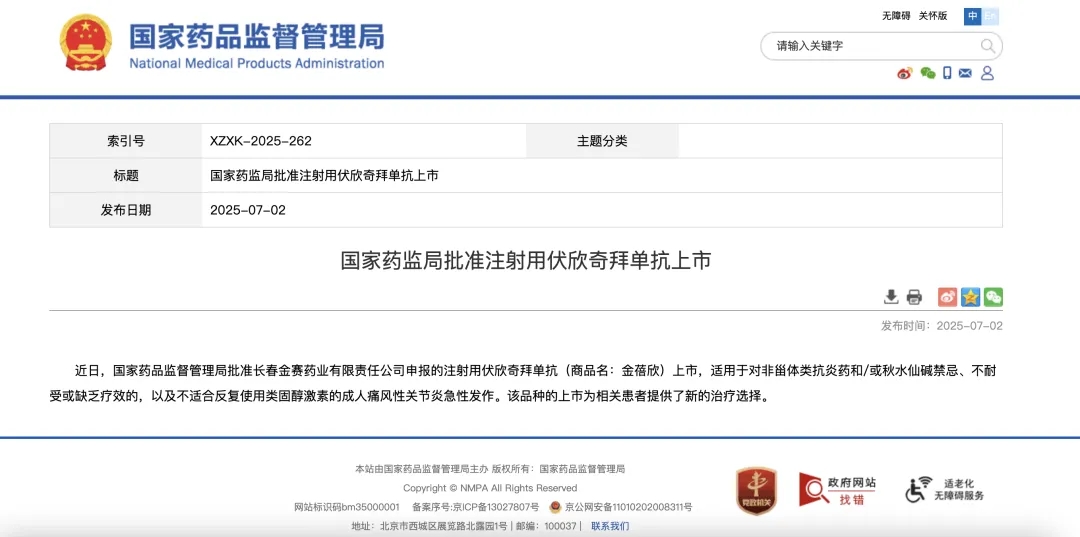
2025-07-04
Firsekibart Approved with 87% Reduction in Gout Recurrence Over 6 MonthsLearn More
-


2025-06-18
NMPA Approves New Indication Clinical Trial of Megestrol Acetate Oral SuspensionLearn More

-
2024-07-03
GenSci Invites you to join us in CPHI South East Asia 2024Join us at Booth H23 from July 10 to 12, 2024 to explore GenSci's flagship products.
Learn More

-
2024-06-28
China's First Aqueous Solution Progesterone Injection, Jin Sai Xin® ApprovedA further step towards GenSci's vision of becoming a global leader in Pediatric and Women's Health
Learn More

-
2024-06-19
GenSci and TWi Reach Exclusive Import and Distribution Agreement for Megaxia®Under this agreement, TWi authorizes GenSci Singapore as the sole distributor of Megaxia® in mainland China, Hong Kong, Macau, and Singapore, responsible for the marketing and promotion of this product.
Learn More

-
2024-06-05
Visit GenSci at CPhI China 2024At Booth W4E59, See you there!
Learn More

-
2024-05-26
NMPA Approves Genakumab Injection for Clinical Trial Commencementwhich marks the initiation of bioequivalence studies for the new formulation of Genakumab Injection
Learn More

-
2024-05-17
Jintrolong® PEG-rhGH from GenSci highlighted at ECE 2024Jintrolong® PEG-SOMATROPIN was highlighted during the Growth Hormone Research Society (GRS) symposium at the largest endocrine event in Europe
Learn More











